Zach has been compensated by Bayer to share his story and experience with Jivi.
Watch More From Zach
Meet Zach or other patients and caregivers at a live speaking event in your area. Hear their unique stories and connect one-on-one.
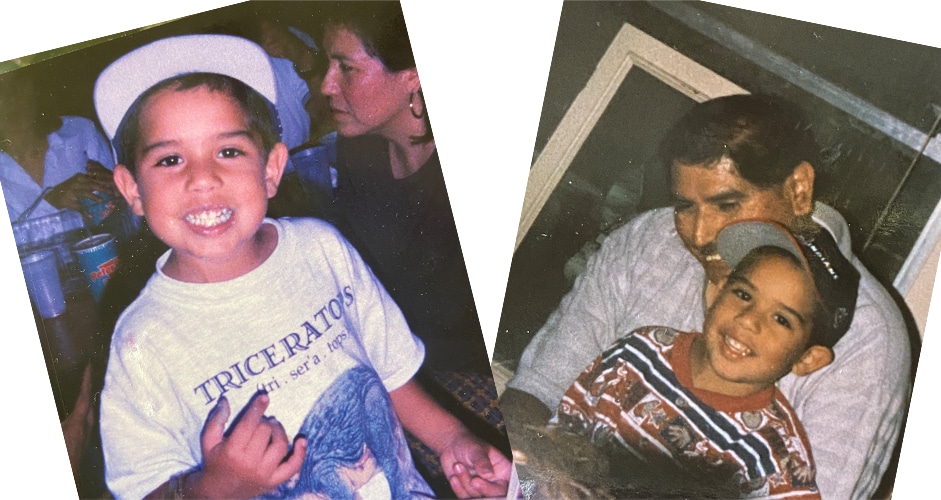





Hear more from patients and caregivers by attending a My Journey to Jivi event.
Meet other Jivi speakers




INDICATION
JIVI is an injectable medicine used to replace clotting factor (Factor VIII or antihemophilic factor) that is missing in people with hemophilia A.
JIVI is used to treat and control bleeding in previously treated adults and children 7 years of age and older with hemophilia A. Your healthcare provider may also give you JIVI when you have surgery. JIVI can reduce the number of bleeding episodes in adults and children 7 years of age and older with hemophilia A when used regularly (prophylaxis).
JIVI is not for use in children below 7 years of age or in previously untreated patients.
JIVI is not used to treat von Willebrand disease.
IMPORTANT SAFETY INFORMATION
You should not use JIVI if you are allergic to rodents (like mice and hamsters) or to any ingredients in JIVI.
Allergic reactions may occur with JIVI. Call your healthcare provider right away and stop treatment if you get tightness of the chest or throat, dizziness, decrease in blood pressure, or nausea. Allergic reactions to polyethylene glycol (PEG), a component of JIVI, are possible.
Your body can make antibodies, called “inhibitors”, against JIVI, or certain components of JIVI, such as polyethylene glycol (PEG), which may stop JIVI from working properly. Consult your healthcare provider to make sure you are carefully monitored with blood tests for development of inhibitors to Factor VIII. Tell your healthcare provider if you have been told you have inhibitors to Factor VIII.
If your bleeding is not being controlled with your usual dose of JIVI, consult your doctor immediately. You may have developed Factor VIII inhibitors or antibodies to PEG and your doctor may carry out tests to confirm this.
The common side effects of JIVI are headache, fever, cough, and abdominal pain.
These are not all the possible side effects with JIVI. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Tell your healthcare provider about all your medical conditions that you have or had.
For additional important risk and use information, please see the full Prescribing Information.
References: 1. Jivi ® Prescribing Information. Whippany, NJ: Bayer LLC; 2018. 2. Data on file. Tx Review 0918. Bayer; 2018.






